Medical Devices
Medical Devices
Established substance-based medical devices with strong claims
Modern life has redefined the way people approach their health. People are no longer just passive consumers or patients – they are becoming proactive “health managers”. This shift highlights, among others, the growing importance of self-medication, which benefits not only to individuals but also the entire economy. In this context, medical devices serve as an excellent and valuable addition to any healthcare-oriented portfolio.
What characterizes Medical Devices?
Medical devices are, by definition, intended for the diagnosis and treatment of diseases. Unlike pharmaceuticals, they do not achieve their effects through pharmacological, immunological, or metabolic processes, but instead work purely through physical or mechanical means.
Substance-based medical devices represent another special category, as they often resemble medicinal products in terms of form and presentation. They meet the definition of medical devices because they are used for disease treatment. Examples include lozenges, nasal sprays, or ultrasound gel.
Choosing our medical devices comes with clear advantages:
- Strong, disease-related product claims
- Uniform regulation across the EU
- Greater differentiation and better protection of intellectual property (e.g., formulation, product claims) due to the time- and cost-intensive EU registration
- Fewer competitors on the market compared to less regulated product categories, such as food supplements

Use our Ready-to-Sell Medical Devices
Our Group member Euro Vital Pharma (Germany) provides a comprehensive portfolio of substance-based medical devices (own IPs). With this portfolio we are a leading supplier for the biggest German retailer.
You can trade our IPs as a co-distributor also outside Germany – we support you in all regulatory matters and ensure that our products comply with all relevant regulations. Accordingly, our quality management system meets the requirements of ISO 13485 for medical devices.

Moreover, as part of the MDR (Medical Device Regulation) transition, we are already fully compliant with the necessary regulations through the 2028 transition period.
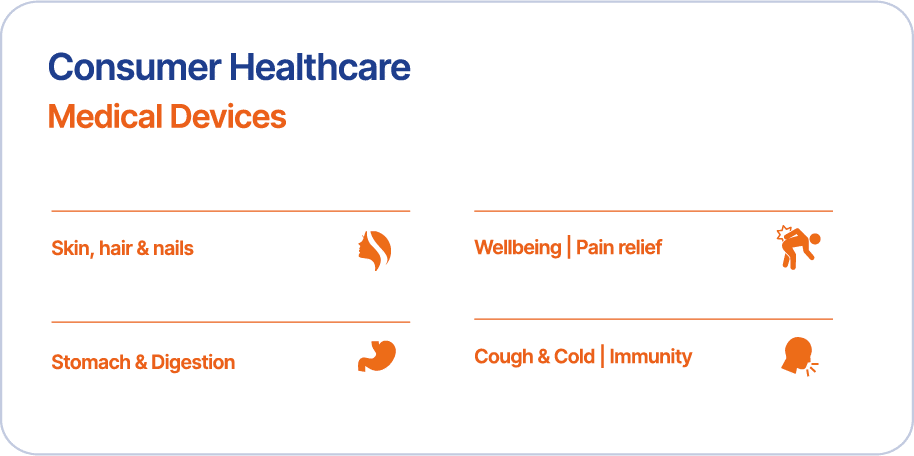
Our Portfolio for Key Health Indications
Our substance-based medical devices with strong claims for oral ingestion, topical application or nasal rinsing, deliver effective solutions in key indications such as:
Cough & Cold, Immunity
- Nasal Spray 20ml (several variants)
- Sea Water Nasal Rinse Spray 100ml
- Nasal Douche Salt (+Nasal Douche Device)
- Nose Cream
- Cough Pastilles Ribwort (+Manuka)
- Sore Throat Salt Pastilles
- Cough Suppressant Islandic Moss (+Honey)
- Sore Throat Spray Natural Brine & Icelandic Moss
Stomach & Digestion
- Simeticon 110 mg Gastrointestional Pastilles
Wellbeing/Pain-Relief
- Headache Roll-On
Skin, hair & nails
- Cooling Gel (minor burns and insect bites)
- Ear Cleansing Spray
- Wart Stop Solution